Future developments in proton therapy
Advances in proton therapy continue unabated. Elsewhere I discuss circumstances that have delayed the public acceptance of proton therapy (see ISSUES), but these do not apply to the steady march of technological and procedural innovation.
COST
Early mistakes of being too large and too expensive now are being corrected by reducing the size of the equipment (and the buildings holding it) through miniaturization and the development of superconducting magnets.
Because protons all have a positive charge, they naturally repel each other. To form a beam requires extremely strong magnets to force them into proper configuration. Cyclotrons also contain massive magnets to speed up the protons to 100,000 miles per second. These magnets draw enormous amounts of electricity (and heat), costing some centers millions of dollars a month.
One solution comes in the shape of superconducting magnets. Encased in highly insulated containers, the magnets register at three degrees Kelvin (about 450 degrees below zero Fahrenheit). The low temperature reduces electrical resistance to almost nil. For example, superconducting magnets developed by Pronova draw four watts! Four! (Compare to a 60-watt light bulb.) Superconducting cyclotrons will follow soon, significantly reducing the cost of producing proton therapy.
Gantries for proton therapy are huge, three stories tall with a mechanism to turn it. (See PHYSICS). The largest gantry I have seen comes from Heidelberg University in Germany, pictured below. Note the size of the person standing under it.

One manufacturer, Mevion, makes a small gantry that fits nicely into a single treatment room. Other manufacturers offer their versions of a smaller footprint.
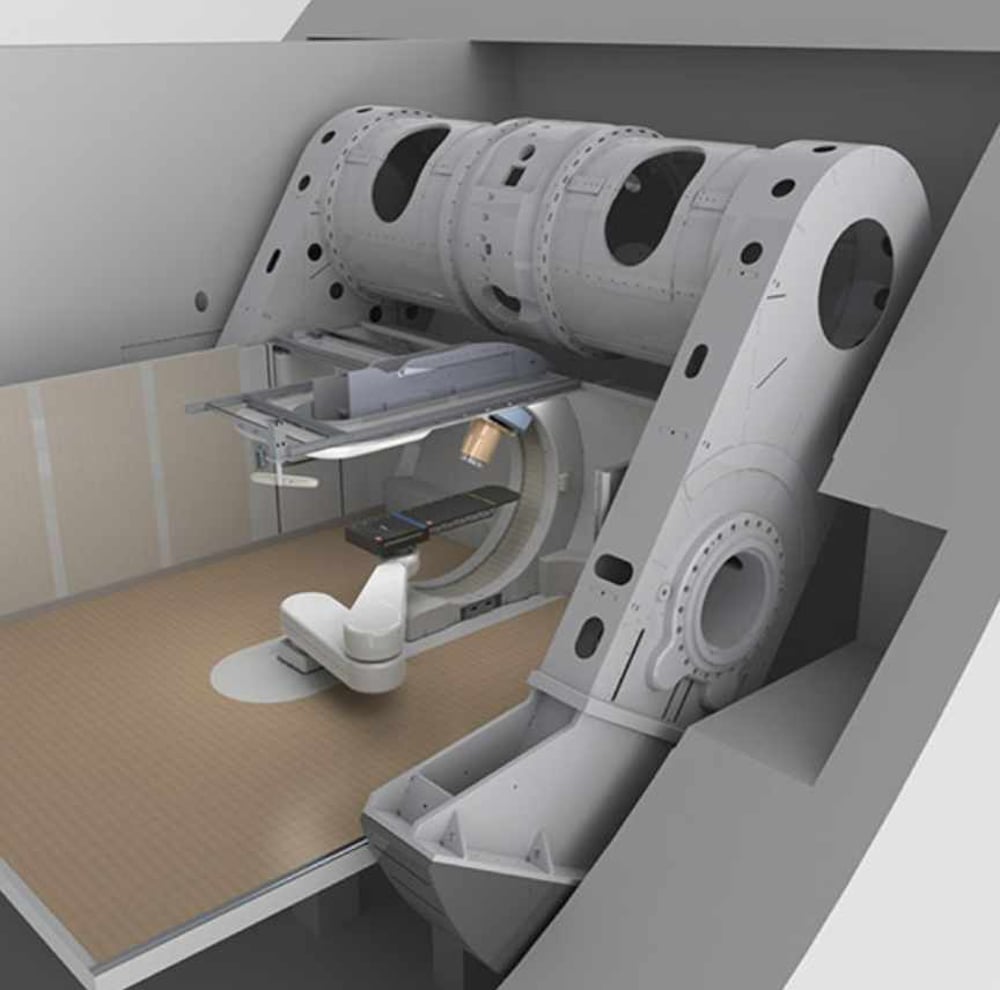
Above: The Mevion S250i proton delivery system. The patient lies on the black table. A ceiling would hide the view of the gantry.
Investments to build a proton center, formerly topping $200 million, now cost one-fifth of that amount. Except for the largest players, all new centers for the past several years have started with a single treatment room. Then, once the single room reaches comfortable profitability, the center can enlarge their facilities by one or two additional rooms.
A study coming from Mass General determined stationary fixed beam proton machines can treat 67% of all cancers rather that using a huge circular gantry. Some smaller gantries move only a partial arc and not a whole circle. Not having a gantry at all cuts the cost by more than half. My prostate cancer was treated on a fixed beam machine the first time and a gantry the second time. A company in Illinois sells a system in which the patient sits upright and a computer moves the patient rather than the gantry.
While the cost of building and equipping a center rapidly declines, operational procedures need to follow.
TREATMENT MODALITIES
The x-ray technology called stereotactic body radiation therapy (SBRT), claims superiority over protons because it requires fewer treatment sessions (see ISSUES). Such a procedure is called hypofractionation. A fraction in a treatment session. Hypo comes from the Greek for “under” or “less than.” Hypofractionation involves reducing the number of sessions while increasing the dose of radiation.
Unfortunately for x-rays, all proton therapy centers offer this procedure, too. Studies show little if any increase in adverse effects. In the first edition of my proton books some four years ago, I mentioned protocols for prostate cancer involving only five treatments. The Mayo Clinic, for example, includes hypofractionation in its array of modalities.
With hypofractionation, canters can treat more patients at a lower cost. Both the proton centers and the insurance companies should be happy about that. And the patients, too, taking fewer days away from home, loved ones, and jobs. The ultimate hypofractionation comprises a single treatment, called flash. More about that shortly.
Combining more than one modality strives to increase the curative benefit of the treatment. Chemoradiation, for example, combines chemotherapy with radiation. Radiation often follows prostatectomy (prostate surgery), to kill any errant cancer cells. Hormone therapy prior to radiation for prostate cancer remains a common combination. The few studies that I have read about combining radiation with other modalities have found proton therapy to be superior to x-rays. Fortunately, I have no personal experience in this area.
The software controlling proton therapy also continues to evolve. Artificial intelligence typically “reads” x-rays and scans of various types better than humans, finding tiny amounts of cancer not visible to the human eye. Software can run hundreds of theoretical designs for radiation treatment, adjusting doses and angles and other variables, from which the dosimetrist chooses the most efficacious plan. Such planning once took days, but now a few hours.
TECHNICAL ADVANCES
Flash represents the most exciting prospect for future treatments. I received 39 proton therapy treatments of 2Gy each, for a total of 78 Gy. (Gy stands for gray, a measurement of radiation.) A hypofractionated protocol for the same cancer may call for five fractions of 5Gy totaling 25Gy.
Flash involves a single treatment, in less than one second, with an extremely high dose, say, 140Gy. It would seem that such a powerful dose could wreak havoc on healthy tissue, but actually, just the opposite seems to pertain. The exact reason remains elusive, but a leading theory involves rapid depletion of oxygen.
Around the world, various centers have joined to study flash. Current proton equipment does not reach the power needed for flash, so this new procedure requires a modification in the technology. Most trials have been performed on animals (beneficially), with one successful human trial complete.
They measure the power of radiation equipment in electron volts. MeV means a million electron volts. (For a thorough tutorial about radiation, see https://webfiles.ehs.ufl.edu/rssc_stdy_chp_2.pdf.) Recently, I read of an equipment manufacturer whose proton equipment could reach 400 MeV. Previously, equipment reached around 250 MeV. The earliest proton center (for treating ocular cancers) reached only 70 MeV since they do not require penetrating the body to any great depth.
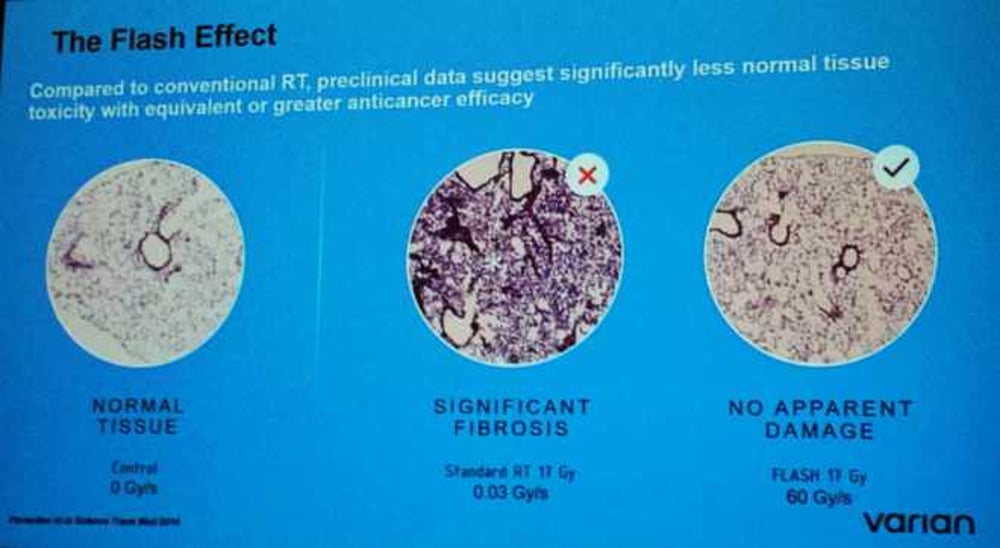
Above: I took this photo at a lecture on flash from a presentation slide. On the left, note the healthy tissue. In the center, the tissue shows damage from a normal radiation routine. On the right the slide reflects a flash treatment, with tissue looking very much like the slide on the left, the healthy tissue, with no damage.
In April, 2019, the University of Maryland proton therapy center unveiled lung cancer research using flash therapy. They reported 25-30 percent less damage to lung tissue, leading to less fibrosis of the lung, and an average 35 percent decrease in skin dermatitis when using flash. They used a hefty dose yet experienced fewer side effects. In their report, they said: “If successful, we could treat patients in a fraction of a second, instead of in seven weeks, with less radiation-induced complications, and with a very high probability to obtain tumor control.” One doctor said, “This may be one of the most exciting developments I’ve seen in my career.”
Presumably one may also use x-rays for flash techniques. One linac machine for that purpose (linac = linear accelerator, used to produce x-rays) reached 10 MeV, five percent the strength of proton beams. Plus, of course, x-rays don’t stop; so I worry about exposure of healthy tissue. If that turns out not to be a problem, both protons and x-rays will benefit, which we all should celebrate.
Since x-ray technology dates back decades, when proton therapy first began it adopted many procedures from traditional radiation. Then, techniques for the two types of radiation diverged based on protons’ unique characteristics. A much-hyped clinical trial purportedly showing x-rays had fewer adverse effects than protons used protons as if they were x-rays, not taking into consideration their greatest strengths (see ISSUES).
Proton therapy typically beams radiation from one, two or three directions. The most recent x-ray equipment beams from 360 degrees all around the body exposing healthy tissue to small doses while the x-rays converge on the target, which accumulates the full dose.
Now comes SPArc, proton therapy delivered beams from a moving gantry that goes around the patient, much like x-rays (but for a different reason). Equipment manufacturer IBA and the proton center in Beaumont, Michigan, have conducted experiments in which the gantry rotates around the patient beaming protons the whole way.
The dosimetry must be a challenge. SPArc produces better results than pencil beam scanning from a single direction. Whereas pencil beam scanning for a brain tumor takes 11 minutes, using SPArc took only four minutes and with 30% smaller dose.
The least accurate part of proton therapy involves stopping at the furthest (distal) point. The sides of the target present much less of a problem. A rotating gantry produces side-like accuracy all the way around.
Although developed more than 70 years ago, progress in proton therapy did not advance until imaging improved. The better the images, the more accurate the treatment. In describing my experience, I include half a dozen ways in which the treatment plan assured maximum accuracy (see EXPERIENCE). One procedure involves taking an x-ray before every treatment. The technicians then superimpose this x-ray over the original one taken during the set-up, making sure they coincide.
Improvements in imaging include cone beam technology (x-rays) built right into the proton equipment.
CT (computed tomography) scans work by rotating x-rays around the body and then using a computer to weave them together into a three-dimensional image. I experienced a new scan called a PSMA scan that combined a CT scan with a radionuclide (radioactive drug) that clearly marked the location of my prostate cancer whereas a standard MRI showed nothing.
Protons prove to be more accurate than x-rays because of their mass. Proton tomography will use protons rather than x-rays during a treatment session. One type of proton tomography developed by ProTom International requires 350 Mev of energy produced by a synchrotron, which works differently than a cyclotron. Flash-qualified cyclotrons could also do it.
Pencil beam scanning (PBS) represents an enormous advance in accuracy, increasing the types of cancers treated from 20% to 80%. When choosing a proton center, I suggest making sure you will receive PBS (see CHOOSING A CENTER). PBS lays down tiny dots in layers and rows. My prostate cancer treatment used more than 5,000 dots. But circular dots have a problem making a straight line, which Mevion addresses in its multi-leaf collimator (MLC), a device that shapes the proton beam much the same way an aperture shapes the earlier double-scattered proton beams (see PHYSICS). A MLC consists of "leaves" of a high atomic numbered material, usually tungsten, that can move independently in and out of the path of the protons, blocking or shaping individual spots.
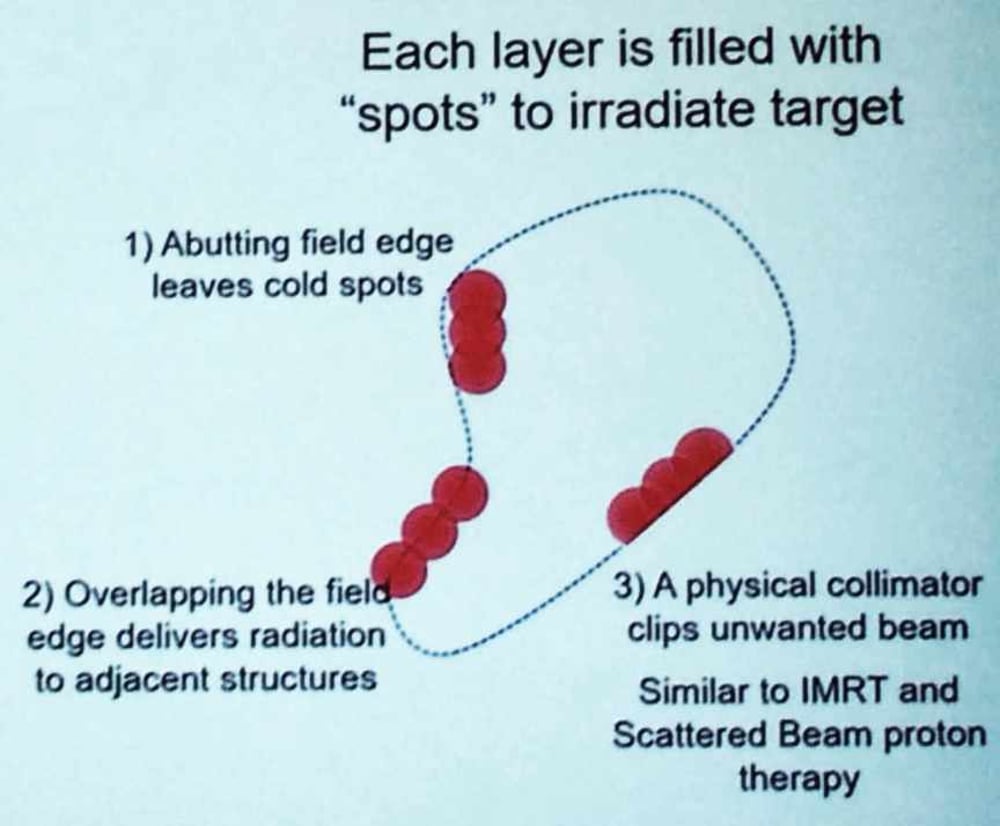
Above: Note how shaping the circles allows a better fit to the target. I think this should be standard equipment.
DEVELOPMENT
The year 2015 represents the height of development and predicted demand for proton therapy. Since 2018 annual reports show a lessening demand. I think they got it right at the beginning when estimating the demand, but unexpectedly various roadblocks slowed things down (see ISSUES).
In late December, 2019, on a website called News Cast Report, I found a seven-year prediction for proton therapy from a company called Data Bridge.
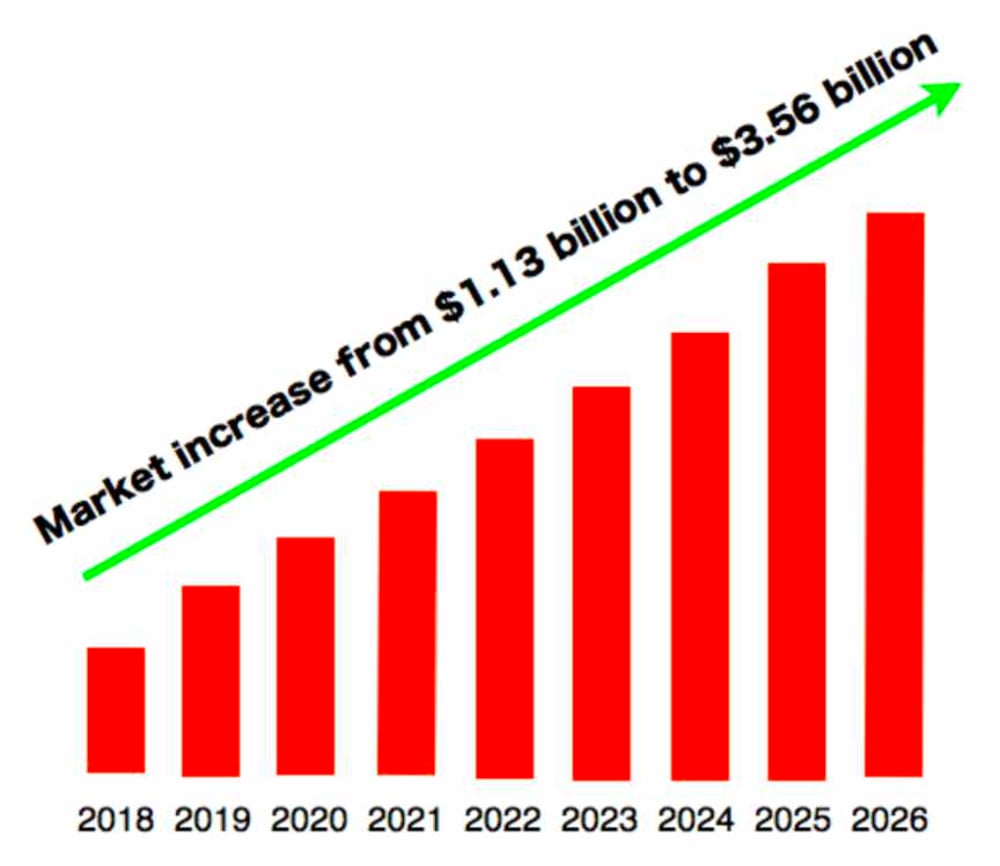
This chart seems suspiciously uniform. I suspect they started with the first year and the last year and just averaged out the years in between. They optimistically estimate triple growth by 2026. A different research company gave the total revenue for 2022 as one billion dollars, which doesn’t coincide with the above graph. Global Market Post predicts the proton therapy market to reach revenue of $2.51 billion by 2028 up from $1.32 billion from 2020, growing at a rate of 13% per year for the next eight years. Still, that predicts less growth than the above chart.
Most of the earliest proton centers have upgraded and expanded to include pencil beam scanning. For some, such as the Mayo Clinics, MD Anderson, and the University of Pennsylvania, demand exceeds capacity, leading them to major expansions.
While I have outlined forces that have hindered the growth of proton therapy (see ISSUES), many of those forces are lessening as protons reach wider acceptance, insurance coverage becomes more inclusive, and costs go down. My list of proton therapy centers (see CHOOSING A CENTER) includes 19 centers under planning and development, although several look doubtful. Certainly more would be in order.
**************
Voila, a partial glimpse of future developments with proton therapy. Some people argue that x-rays have reached the apogee of their development (except, perhaps, for potential flash use) whereas the prospects for proton therapy stretch well into the future. Imagine this. A man with prostate cancer receives a PSMA scan that pinpoints the location of the cancer. Then he receives a single treatment using flash protocol. That’s it. All done. Virtually no side effects encountered. He goes back to his life in a matter of days. Both PSMA and flash will make such a scenario possible.
Personally, I hope proton therapy replaces chemotherapy and its attendant outrageous cost, side effects and suffering. Whatever the case, proton therapy represents the future of cancer treatment. Another frontier, lifestyle and diet, could eliminate cancer. Wouldn’t it be great if there were no need for proton therapy? But I’ll save that for a different website.
RETURN TO PROTON THERAPY PAGE
GO TO HOME PAGE